2.4.1 Chemistry, Types and Characteristics of CBN
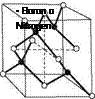
The invention of the superabrasive cubic boron nitride (CBN) is linked closely to the synthesis of artificial diamond. CBN has the same crystal structure as diamond (cubic zinc blende structure), but the carbon atoms are replaced by the elements boron and nitrogen (Fig. 2.19 left). Boron nitride (BN) appears in forms isostructural to carbon: hexagonal a-BN similar to graphite, diamond-like P-BN, y-BN in the wurtzite structure, and others [GREI06, HAUB02].
 |
![Подпись: Density 3.47 g/cm3 [MULL01]* Hardness Knoop 42 - 45 GPa [MULL01]* Fracture toughness 3.7 MPa m1/2 [MULL01]* Thermal stability Up to 1200 oC [MULL01]* Thermal conductivity 240 - 1,300 W/m K [ROWE09] *after 3M, GE, Norton, Treibacher](/img/3149/image041.gif) |
In CBN and diamond each of their atoms is bonded to four others in a perfect tetrahedrally alignment (bond angle 109° 28′). In CBN, each nitrogen atom is bonded to four boron atoms and vice versa. Cubic boron nitride has predominantly covalent bonds with a small degree of ionic bonding because boron and nitrogen are dissimilar atoms [BAIL98].
Fig. 2.19 Basic properties of cubic boron nitride
