2.3.3.1 Monocrystalline Diamonds
The first artificial diamond synthesis was conducted by the Swedish company ASEA with a six-anvil press in February 1953 [TOLA68, WEDL77]. In 1955, company GE followed with the synthesis in a belt press and in 1958 company De Beers [MARI04].
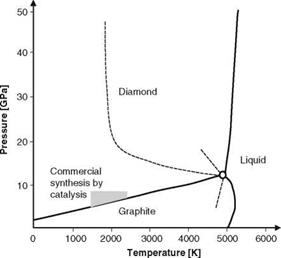
Graphite can be transformed to diamond directly at temperatures above 1800 K and pressures above 60 kbar (Fig. 2.17) [WILK91]. However, the use of appropriate catalysts can reduce the temperature-pressure-range significantly. Metals of the 8th main group (nickel, cobalt, iron, et al.) enable synthesis even at pressures of 5-8 GPa and temperatures of 1500-2100 K [ODON76, YIN00]. This method takes advantage of the fact that graphite solves easier in metal than diamond. Graphite is also less thermodynamically stable within the working range. As consequence, carbon precipitates as diamond from the supersaturated metal carbide solution [HOLL95, MARI04].
|
Fig. 2.17 Pressure-temperature diagram of carbon [BUND96] |
 |
 |
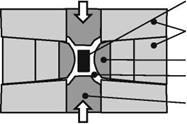 |
Fig. 2.18 Presses for diamond production, left belt-press [HALL60, KOMA01, LUND68, KHVO08, COES71], right anvil press [KHVO08]
together and generate the needed high pressure. Pyrophyllite creeps through the press joints and seals them enlarging the internal pressure in the reaction chamber [LUND68]. Resistor elements in the chamber walls generate the high temperatures needed for diamond synthesis [LUND68]. State of the art belt-presses reach a pressure of 8-10 GPa [KHVO08].
Today, another press type, the six anvil press, is widely used. It has the advantage of reaching higher pressures of 20-30 GPa in relatively large chamber volumes (around 10 mm3) (Fig. 2.18 right). In Russia, diamond synthesis is conducted in chambers of a toroid and lentil type with even higher pressures. [KHVO08]
Quick temperature and pressure changes during diamond synthesis can force segregation of many diamond seeds. They will interfere during growth so that polycrystalline and irregularly grown diamonds develop [ODON76, PIRA03]. Before industrial use, synthetic and natural diamonds are often broken mechanically or prepared with acid to change their surface [TOLA68]. Whereas natural diamond must be crushed before use in grinding tools, diamond synthesis produces abrasive particles in appropriate size for abrasive applications [METZ86, p. 38].