 |
![Подпись: [111] Octahedral planes 16]](/img/3149/image035_0.gif) |
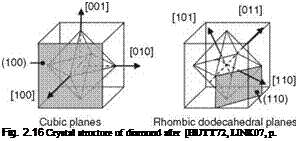
Diamond density is about 3.52 g/cm3 depending on pureness. Diamond hardness and toughness are determined by crystal purity, regardless of size, shape, and genesis [BENE03]. The hardness of single crystal diamond is anisotropic depending on the crystal orientation (Fig. 2.16). This results from the different distances of the carbon atoms in different crystal planes. The highest density of atom bonds occurs in the octahedral plane (111), which results in the highest hardness [TOLA68]. The cubic plane (100) is the softest direction [TOLA68]. In the (110) plane the hardness iis as high as 123 % of the hardness in the (111) plane, in the (100) plane even 138 % [KLOC05a].
Hardness and cleavage behavior are not only defined by the crystallographic structure but also by the occurrence of structural defects and diamond purity [BRUN62, WILK91]. Smaller grits are commonly tougher than bigger diamonds because they have fewer and smaller defects and inclusions [FIEL79, WILK91].
Synthetic diamonds often contain metal inclusions of the catalysts [YIN00]. All crystal defects like substituted atoms, atoms between lattice sites, or lattice vacancies are imperfections of diamond structure and enable micro-splintering [ODON76]. In addition, macroscopic inclusions can act as initial points for crack growth.
