 |
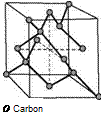
The crystal unit cell of diamond consists of eight carbon atoms (Fig. 2.13). Four of these (8-1/8 + 6-1/2) define a cubic area-centered lattice. The left four atoms are positioned on the centers of the eighth dice and build a second cubic area-centered lattice that is displaced in the direction of the body diagonal with a quarter of its length [HUTT72]. The lattice constant of diamond is a = 0.355 nm. Every carbon atom in diamond is surrounded tetrahedrally by four atoms, in which the angle between two neighboring atoms amounts consistently to 109.5° and the distance is 1.5445 A [HOLL95, HUTT72, MORT87]. These angles originate from the geometrical crystal structure due to the sp3-hybridization: when one of the 2s-electrons is lifted into the empty p-orbit from the basic state (1s22s22p2). From three p-orbits and one s-orbit four new similar orbits are generated. The four hybrid orbits push each other away, so that the orbits are directed into the edges of a regular tetrahedron [HOLL95, MORT87].
|
Density |
3.53 g/cm3 |
[ROWE09] |
|
Hardness Knoop |
65 GPa |
[ROWE09] |
|
Fracture toughness |
3 — 3.7 MPa. mA0.5 |
[GRAN12] |
|
Thermal stability |
500 -700 oC |
[GARD88] |
|
Thermal conductivity |
600 — 2,000 W/m K |
[ROWE09] |
Several diamond materials are used in abrasive tools today [MARI04]:
• Natural diamond grits,
• Synthetic diamond grits,
• Synthetic mono-crystalline diamond logs (MCD),
• Synthetic poly-crystalline diamonds in Co-matrix as logs or plates (PCD) and
• Chemically disposed diamonds without binder as logs or plates (CVD).
