The raw materials are silicon dioxide (SiO2) in the form of white quartz sand with a purity of 97-99.5 % SiO2, and petroleum coke (C) (Fig. 2.11) [LEWI76, p. 8, STAD62, p. 25]. Both materials are ground to particle sizes of 1-2 mm [STAD62, p. 25]. In addition, sawdust and salt are inserted. The sawdust carbonizes during the reaction process and leaves pores, through which the resulting carbon monoxide (CO) can escape (Eq. 2.10) [STAD62, p. 25]. The salt turns impurities such as aluminum or iron to chlorides. An example composition of raw materials is 53 % quartz, 40 % coal, 5 % sawdust, and 2 % salt [KLOC09, p. 34].
Commonly, resistance furnaces are used. The furnace is half filled with the raw material mixture (Fig. 2.12). A central core of petroleum coke, nut-coal or graphite is inserted in the middle of the furnace [LEWI76, p. 8, STAD62, p. 25]. The rest of the quartz, coke, sawdust mixture is put on top. At each end of the oven are electrodes [LEWI76, p. 8]. The central core conducts the electric current and acts as resistor. At a voltage of for example 240 V the current heats the furnace to the reaction temperature of around 2000 °C [STAD62, p. 25]. Coke and sand react after Eq. (2.10) to hexagonal а-SiC [LUDE94]. It has to be noted that SiC production inherently produces CO emissions. The resistor core can be straight or zig-zag-shaped [MOSE80, p. 123].
SiO2 + 3C! SiC + 2CO (2.10)
At higher temperatures the core resistance drops and the reaction mass begins to conduct, so that the applied voltage and power input have to be controlled [LEWI76, p. 8, STAD62, p. 25]. The voltage is reduced so that the furnace has a constant power consumption of for example 1000 or 3000 kW [STAD62, p. 25].
|
|
|
|||
|
|
||
|
|||
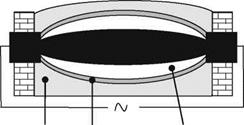
Fig. 2.12 Cross-section of a resistance furnace for silicon carbide production after [STAD62, p. 25; TYRO03b, BORC07, p. 49 ff]
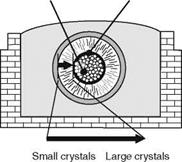
The energy per kg of a-SiC produced is around 8 kWh/kg [TYRO03b]. The reaction ends after 36-40 h and results in several reaction products (Fig. 2.12).
After cooling and stripping the furnace walls, unreacted mass is obtained that can be used for further reactions [LEWI76, p. 8]. The ingot of silicon carbide is surrounded by a hard crust consisting of salt and silicon carbide dioxide. The crystals in the silicon carbide layer increase in size and purity from outside to inside; also, the color of the crystals shifts from black to green [STAD62, p. 25]. Graphite from decomposed SiC surrounds the electrode core [LIET08].
Different grades of pureness, toughness, and color can be produced depending on the process control [LEWI76, p. 8]. Like in corundum production, the material blocks are crushed and milled to the according size (see Sect. 2.6 “Grit Post-Processing”).
Waste from the manufacturing process is unreacted coke and partly reduced “firesand”, which can be returned to the furnace [MALK08, p. 24 f.]. In some furnaces, the gases from the reaction are collected and used for energy production [LIET08].