Oil-in-water emulsions are used when what matters is a good cooling effect and not as much the lubrication effect. For oil-in-water emulsions, an even distribution of extremely fine oil droplets in water is obtained by means of so-called emulsifiers. Since the physical properties of emulsions is largely the same as that of water — the amount of oil is usually considerably less than 10 % — their specific heat capacity is about twice as high and the heat conductivity four times higher than that of oil (table 5-1). In accordance with the size of the oil drops, we differentiate between
• coarsely-dispersed emulsions (average oil drop size between 1 und 10 |im),
• finely-dispersed emulsions (oil drop size between 0.01 und 1 |im),
• colloidally-dispersed emulsions (oil drop size between 0.01 und 0.001 |im) and
• molecularly-dispersed systems (drop size under 0.001 |im) [ZWIN60].
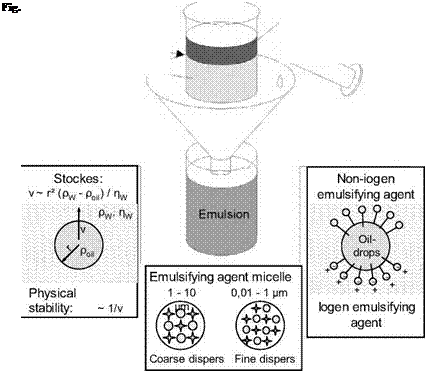 |
This size of the oil drop formed from emulsification is dependent above all on the type and amount of emulsifier. Increasing the amount of emulsifier lowers the droplet size (Fig. 5-4).
The oil drop size is of particular importance, as it has decisive influence on the physical stability of the emulsion. According to Stockes’ law, the climbing speed of the oil droplet, which is inversely proportional to stability, increases with the square of the radius and the difference in density. The viscosity of the surrounding phase (water), on the other hand, works in the opposite way. Since particle size comes into play to the greatest extent in emulsion stability, most stabilising measures applied to metal machining emulsions strive to increase the degree of dispersion by adding emulsifier concentrates.
Also with respect to resistance to microorganisms, which affect all water-based products, finely-dispersed emulsions show better performance than coarsely — dispersed ones.
Coarsely-dispersed emulsions are milky and opaque. With decreasing drop sizes, the fluid’s transparency increases. When using an emulsion, the concentrate should be added to the water by means of a dosing apparatus or alternatively in a
thin jet under constant stirring and not the other way round, as otherwise soap agglomerations and non-emulsified concentrate inclusions could result.
The properties of the water have a large influence on the quality and operational behaviour of emulsions. The water used must be very pure. Well or river water often introduces considerable amounts of bacteria into the unused emulsion. But the excessive chloride content of tap water also compromises stability and corrosion protection properties. Above all, the mixing water’s nitrate content should be considered, as nitrate (NO"3) can be decomposed chemically or bacterially to nitrite (NO"2) [LING93]. As a nitrosation agent reacting with amines, nitrite leads to the development of N-nitrosodiethanolamine (NDELA), which is considered carcinogenic. Water hardness should be about 5 to 10° measured in accordance with the German water hardness system. Water that is too soft amplifies foam-formation, while excessively hard water leads to foam separation due to reactions with hardening agents. The water used should have a pH-value of 6 to 7 (neutral) and the emulsion between 8 and 9.5 (slightly alkaline). If the value is lower, the risk of corrosion increases.
