In oxidic glasses, both covalent and ionic bonds predominate. They generally display strong polarisation, which also causes its characteristically brittle removal behaviour. Should material shifting lead to the fracture of these bonds, the structure of glass is destroyed irreparably as opposed to metals, the free electrons of which can form new bonds.
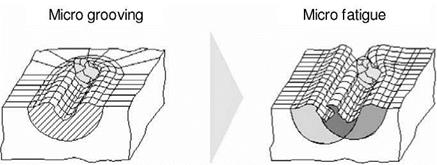
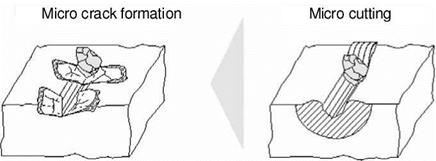
Figure 4-18 shows a schematic depiction of the material behaviour of glasses during when penetrated by an abrasive cutting edge with increasing chip thickness. Until exceeding the critical chip thickness, which initiates micro-cracks, a ductile machining process is also realisable for these materials — despite their amorphous structure. The creation of damage-free external component areas is also possible for glasses by means of the machining mechanism.
|
|
|
Fig. 4-18. Possible removal mechanisms in the engagement of a grit with glass |
It is assumed that highly negative cutting angle needs to be employed for ductile material removal in order to obtain a condition of hydrostatic compressive stress. Beyond that, several further measures are helpful in reducing individual chip thickness, such as:
• the use of smaller granulations,
• higher grain concentrations,
• low feed rates,
• low depths of cut,
• high cutting speeds (as long as thermal damage is avoided),
• high radial accuracy of the grinding wheel (the use of ultra-precision machines is required),
• an even envelope (no exposed grains) [KOCH91].