Metallic Titanium
Titanium and titanium alloys have a low density (p = 4.5 g/cm3) and high tensile strength (Rm = 900 — 1400 N/mm2). They exhibit good heat resistance up to temperatures of ca. 500 °C. In addition, they are resistant to many corrosive media.
From these properties are derived the main applications of titanium material, these being in air and space travel and the chemical industry. More universal use is prevented by the price, which is several times higher than steels and aluminium alloys.
Titanium materials are subdivided into four groups (table 4-7):
I. Pure titanium,
II. a-alloys,
III. (a + P)-alloys and
IV. P-alloys
Pure titanium varieties, also called unalloyed titanium, contain small amounts of oxygen, carbon, nitrogen and iron. Their strength can be enhanced by adding up to 0.45 % oxygen. Their resistance to corrosion can be increased by adding palladium (max. 0.2 %).
Titanium is found at room temperature in the hexagonal a-modification. This transforms at 882.5 °C into the space-centred cubic P-modification. By adding alloying elements, the P — a — transformation can be pushed to low temperatures so that the P-phase remain stable at room temperature and below.
a-alloys contain aluminium, tin and zircon as their main alloying elements. Additional elements are vanadium, silicon, copper and molybdenum (max. 1 %). Cupriferous alloys are hardenable.
P-alloys contain vanadium, molybdenum, manganese, chrome, copper and iron. Vanadium and molybdenum form with titanium a continuous series of mixed crystals, which remain stable even at low temperatures. Mixed crystals with the other
![]()
|
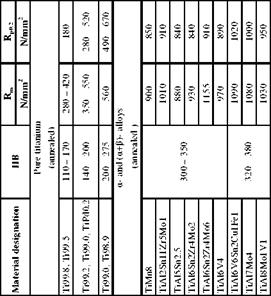
(a + P)-alloys contain the alloying elements of both alloy groups mentioned above. These bimodal alloys exhibit higher strengths than the single-phased a — alloys. They can be hardened more strongly and are suited for use in high temperatures [ZWIC74].
