Corundum has the comparatively lowest thermal conductivity of the common abrasives with X = 6 W/m °C for brown corundum [KLOC09, p. 27]. This leads to a comparatively higher heat flux into the workpiece compared to heat flux into the grit material during grinding [BRIN82, p. 128].
2.1.2 Manufacture of Corundum by Electrofusing
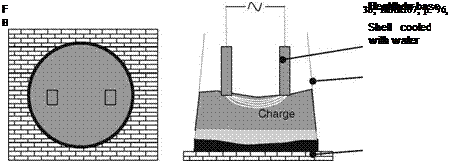
The Higgins furnace consists of a metal shell on a heavy metal hearth (Fig. 2.4). [MARI07, p. 76, HIGG04]. The shell builds the crucible and can be moved up and down relative to the electrodes, which are inserted into the raw materials. An arc builds between the electrodes through the processed material. The shell and bottom are water-cooled.
Melting times depend on the applied method and the furnace size (block furnace/Higgins furnace 15-24 h, tilting furnaces 3-5 h) [KLOC05a, p. 23]. The subsequent cooling and solidification of the molten mass also depends on the oven size (4-20 tons weight) and can take up to 14 days [ENGE02, p. 6, KLOC05a, p. 24]. The size of the corundum crystals depends on the block size and cooling rate and can range from 0.2 mm to several millimetres [ENGE02, p. 6].
The cooling procedure happens either through the billet method or the tilting method. In the billet method, a huge block of up to 20 tons cools down slowly in air, which takes up to 10-14 days, and large crystals are formed. In the tilting method, the molten mass cools in flat casting pans. Fine crystals result from the quicker cooling.
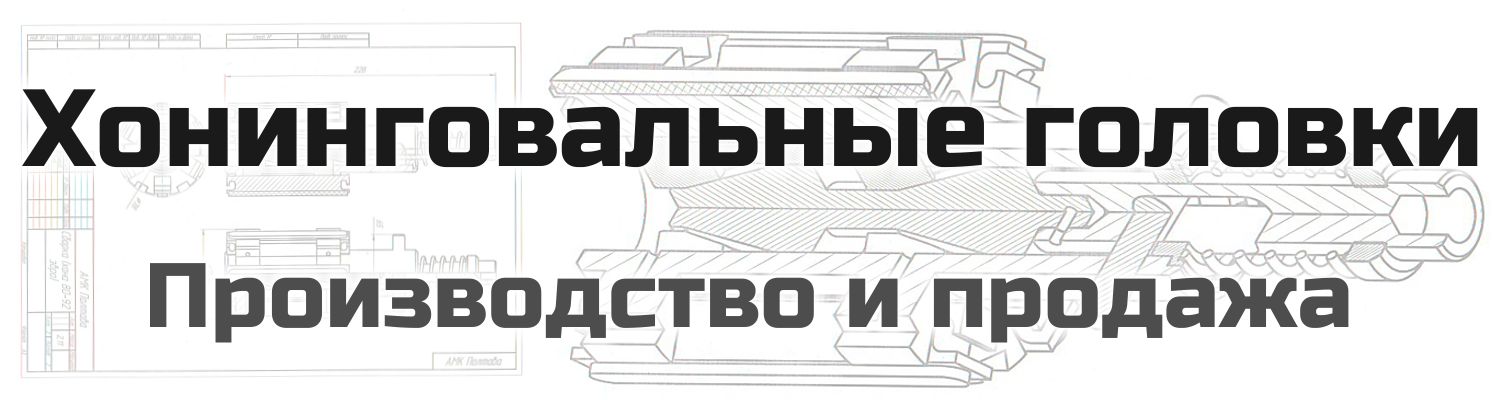
The main raw material, bauxite (Al2O3 + SiO2 + TiO2 + Fe2O3), is an impure aluminum oxide found in nature. The name refers to Les Baux in France where it was first quarried [LEWI76, p. 9]. Natural Bauxite contains 80-90 % alumina with impurities such as 2-5 % TiO2, and Fe2O3, Fe(OH)3, silicic acid, Al(OH)3, and aluminum oxide hydrates [KLOC05a, p. 23, MARI07, p. 76]. Before fusion, the bauxite is either calcinated or purified by the Bayer-process.
Changes in the ingredients or in the fusion procedure result in several different types of fused or molten corundum as follows: Brown and semi-friable corundum is made from calcinated bauxite (Fig. 2.5) [KLOC05a, p. 23, LEWI76, p. 9]. The calcination process takes place at about 950 °C and dehydrates the bauxite [LEWI76, p. 9]. Around 80 % calcined bauxite, 16 % iron and 4 % coke are used [KLOC09, p. 21]. The fusion of 1 ton of of brown corundum needs around
2.2 MVA [JACK07, p. 24].
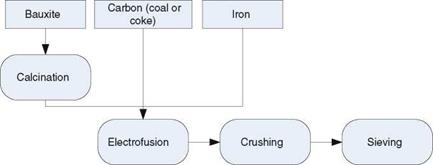
White corundum is produced from relatively pure raw materials, in particular from >99 % pure alumina (Fig. 2.6) [MARI07, p. 77]. These pure clays of Al2O3
|
|
|
Fig. 2.6 Production of white, pink or ruby corundum after [JACK11, p. 22] |
are produced from Bauxite by the Bayer-method [KLOC05a, p. 23]. Fusion of 1 ton of white corundum needs around 1.5 MVA [JACK07, p. 24]. Due to a small amount of sodium oxide, this grit type is friable and “cool cutting” [LEWI76,
p. 10].
Pink corundum and ruby corundum are made like white corundum, but chromium oxide Cr2O3 is added in amounts of 0.3 or 2 % respectively to the fusion process (Fig. 2.6) [KLOC05a, p. 27]. The chromium oxide is built into the Al2O3 crystal structure. Additions of vanadium oxide give a green corundum abrasive [LEWI76, p. 10].
Mono-crystalline corundum is produced like white corundum, but iron sulphide is added besides coke to the molten mass, so that a pure, mono-crystalline aluminium oxide forms in a ferrous sulphide matrix [KLOC05a, p. 27, TYRO03b]. The Na-content is beneficially low [TYRO03b]. The sulphidic matrix is crushed and treated with water so that the monocrystalline corundum grits are washed out [MALK08, p. 23]. The crystal structure is defectless and, therefore, a higher toughness than conventionally molten corundum is obtained [ENGE02, p. 6]. In addition, no high crushing forces are used to acquire the grits [MALK08, p. 23]. Environmental complications accompany the process [TYRO03b].
A more or less regular, spherical form characterizes hollow sphere corundum [KLOC05a, p. 27 f.]. It can be obtained from different manufacturing routes. One way is to melt the raw materials in an electric arc oven and pour the material out of the furnace through a high-pressure air stream [HAUE07]. The molten stream transforms into smaller particles, which cool while flying through the air. Surface tension forces the particles into spheres. The exterior of the spheres cool quicker than the inside, so that material shrinks away from the sphere center and leaves an open core [HAUE07]. The high cooling rate results in a fine-crystalline structure [HAUE07]. The air stream velocity controls the sphere size between 5 mm to 100 |jm [HAUE07].
Zirconium corundum is generated through adding of up to 40 % zirconium dioxide ZrO2 to the fusion process and a subsequent chill casting [WASH12b]. During solidification, eutectic structures of Al2O3 and ZrO2 are formed [KLOC05a,
p. 27]. Mechanical load or heating of up to 700 °C induces a transformation of the zirconium oxide from the tetragonal into the monocline phase. Herein, the oxide volume increases and micro-cracks build inside the abrasive grit, which inhibit cracks under load [ENGE02, p. 7]. The wear mechanism of zirconium corundum can be influenced by the content of ZrO2 to a certain degree. A decreasing ZrO2 content results in falling toughness and the breakage of bigger grit particles as a consequence, but the tendency towards larger wear areas by abrasion is also diminished [LUDE94, p. 99]. Zirconium corundum grits cannot be used in vitrified bonds because the sintering process with temperatures above 900 °C leads to destruction of the abrasives by volumetric change [ENGE02, p. 7].