CBN is thermally stable in nitrogen or vacuum to at least 1,500°C. In air or oxygen, CBN forms a passive layer of B2O3 on the surface, which prevents further oxidation up to 1,300°C. However, this layer is reactive with water, or more accurately high temperature steam at 900°C, and will allow further oxidation of the CBN grains following the reactions [Carius 1989, Yang, Kim, and Kim 1993]
2BN + 3H2O ^ B2O3 + 2NH3 BN + 3H2O ^ H3BO3 + N2 > 900°C
4BN + 3O2 ^ 2B2O3 + 2N2 > 980°C
B2O3. + 3H2O ^ 2H2BO3 > 950°C
5.7.8 Effect of Coolant on CBN
Reactivity has been associated with reduced wheel life when grinding in water-based coolants compared with straight-oil coolants. However, the importance of this reaction is not clear-cut as water also inflicts a much higher thermal shock to the crystal as it is heat cycled through the grinding zone. Regardless of root cause, the effect is dramatic as illustrated in Table 5.2, which gives comparative life values for surface grinding with CBN wheels [Carius 2001].
CBN is also reactive toward alkali oxides—not surprising in light of their use as solvents and catalysts in CBN synthesis! The B2O3 layer is particularly prone to attack or dissolution by basic oxides such as Na2O by the reaction
B2O3 + Na2O ^. Na2B2O4 . . . . [GE Superabrasives 1988]
 |
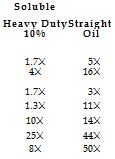 |
Such oxides are common constituents of vitrified bonds and the reactivity can become extreme at temperatures above 900°C affecting processing temperatures for wheels [Yang, Kim, and Kim 1993].
5.7.11 Effect of Reactivity with Workpiece Constituents
CBN does not show any significant reactivity or wetting by transition metals such as iron, nickel, cobalt, or molybdenum until temperatures reach in excess of 1,300°C. This is reflected in a low rate of wear when grinding these materials with CBN abrasive in comparison with wear of diamond abrasive. CBN does show marked wetting by aluminum at only 1050°C and also with titanium. As demonstrated in wetting studies of low temperature silver-titanium eutectics, CBN reacts readily at 1,000°C to form TiB2 and TiN [Benko 1995]. This provides an explanation of why in grinding aerospace titanium alloys such as Ti-6Al-4V, CBN wheels wear typically five times faster than diamond wheels [Kumar 1990]. By comparison, the wear rate using the alternative of SiC abrasive is 40 times greater than CBN. This is a further example of the need to consider the combined effects of the mechanical, chemical, and thermal wear processes as much as abrasive cost.
Pure, stoichiometrically balanced CBN material is colorless, although commercial grades are either a black or an amber color depending on the level and type of dopants present. The black color is believed to be due to an excess (doping) of boron.
5.7.12 Thermal Properties of CBN
The thermal conductivity of CBN is almost as high as that of diamond. At room temperature, thermal conductivity is 200 to 1,300 W/mK, and the transient thermal property в = 2.0 x 104 to 4.8 x 104 J/m2sK. The thermal expansion of CBN is about 20% higher than diamond.
