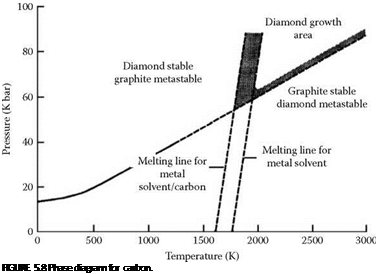
Diamond is created by the application of extreme high temperatures and pressures to graphite. Such conditions occur naturally at depths of 120 miles in the upper mantle or in heavy meteorite impacts. Diamond is mined from Kimberlite pipes that are the remnant of small volcanic fissures typically 2 to 50 m in diameter where magma has welled up in the past. Major producing areas of the world include South Africa, West Africa (Angola, Tanzania, Zaire, Sierra Leone), South America (Brazil, Venezuela), India, Russia (Ural Mountains), Western Australia, and most recently Canada. Each area, and even each individual pipe, will produce diamonds with distinct characteristics.
3.4.2 Production Costs
Production costs are high, with 13 million tons of ore, on average, processed to produce 1 ton of diamonds. Much of this cost is supported by the demand for diamonds by the jewelry trade. Since World War II, the output of industrial grade diamond has been far outstripped by demand. This spurred the development of synthetic diamond programs initiated in the late 1940s and 1950s [Maillard 1980].
3.4.3
 |
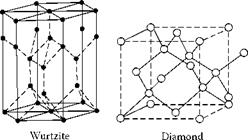 |
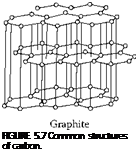
Three Forms of Carbon
 |
The stable form of carbon at room temperature and pressure is graphite, where the carbon atoms are arranged in a layered structure. Within the layer, atoms are positioned in a hexagonal lattice. Each carbon atom is bonded to three others in the same plane with the strong sp3 covalent bonding required for a high hardness material. However, bonding between the layers is weak, being generated from Van de Waals forces only, and results in easy slippage and low friction. (In fact, pure graphite is highly abrasive because, although there is low friction between the layers, the edges of individual sheets have dangling bonds that are highly reactive. It is only the presence of water vapor in the air of dopants added to the graphite that neutralizes these sites and makes graphite a low-friction surface). Diamond, which is meta-stable at room temperature and pressure, has a cubic arrangement of atoms with pure sp3 covalent bonding with each carbon atom bonded to four other carbon atoms. There is also an intermediate material called wurtzite or hexagonal diamond where the hexagonal layer structure of graphite has been distorted above and below the layer planes but not quite to the full cubic structure. The material is nevertheless almost as hard as the cubic form.
